In a COVID-19 world, patient safety and innovation in medical device capabilities have become paramount to saving lives. In this context, the Unique Device Identification (UDI) System as a topic of discussion is garnering increasing attention.
The UDI was introduced in September 2007 as part of the Food and Drug Administration Amendments Act of 2007, and mandates that a unique device identifier label be assigned to medical devices within the US (and Europe). The intent—establish a common vocabulary in the form of human- and machine-readable device labels to enhance electronic tracking, leading to:
- Modernized device surveillance
- Improved patient safety
- Facilitated medical device innovation
From manufacturing through distribution to patient use, the UDI system enables reliable and consistent identification of medical devices by helping:
- Identify counterfeit products
- Improving the ability of staff to distinguish between devices that are similar in appearance but serve different functions
- Facilitate and improve the recall process
- Create efficiencies within the medical system
The following diagram illustrates the data transmission flow of medical devices through healthcare systems:
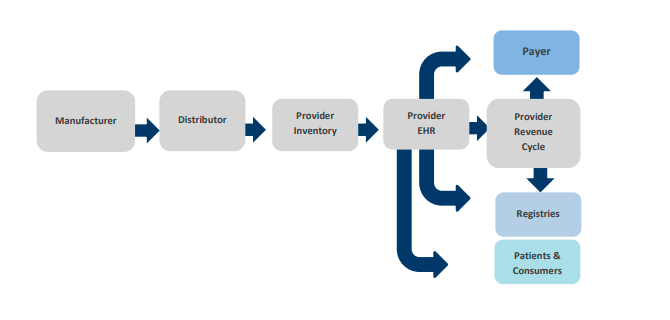 Image source : https://www.fda.gov/media/91988/download
Image source : https://www.fda.gov/media/91988/download
How UDI impacts medical device manufacturers
UDI implementation is not only restricted to the outer packaging of medical devices. Some types of devices require an UDI label to be printed on the body of the device too. This labeling, known as direct marking, helps concerned parties track the device even without the packaging. Direct marking is generally used for devices meant for extensive use after unpacking.
By tracking a medical device through the entirety of a supply chain, UDI empowers patients and healthcare providers to acquire holistic information necessary to make correct critical decisions. That said, implementing the UDI system can pose a few challenges, including:
- Some medical devices are customized according to patients’ needs, and can come under the purview of different regulations — leading to multiple UDIs on a single medical device
- Apart from medical devices, medical device manufacturers are required to print different UDIs for all the accessories of different kinds of devices
- Direct marking comes with its own share of technical challenges such as the differential size of devices and interference with the safety quotient
- Labeling combination products can prove to be ambiguous if the device contains an active pharmaceutical ingredient
To resolve these challenges, companies are actively pursuing technologies that can help them reduce implementation errors and meet FDA mandated unique device identifier compliance requirements.
Digital transformation, medical device manufacturers and UDI
The global Internet of Medical Things (IoMT) market is forecast to touch the $285.5 billion mark by 2029 (having accounted for $24.4 billion in 2019) after registering a CAGR of 28%--a figure the current pandemic is expected to amplify as healthcare systems try to minimize direct exposure between healthcare workers and patients by rolling out contactless solutions.
Integrating more IoT will help healthcare players to streamline their data collection and give them complete visibility into what is happening within their organizations. Implementing the UDI, for instance, is a key step towards realizing this. To quote a leading medical technology journal, “After ensuring that all devices contain an UDI, the process of inventorization of a healthcare system’s medical devices and uploading them to the central database can be carried out more efficiently in batches. Additionally, by ensuring that each device is connected to a network the process of keeping track of the devices in service can be carried out much more efficiently.”
What does the future hold for UDI and medical device manufacturers?
The medical device industry is all too familiar with constantly changing compliance and regulatory requirements. Where UDI implementation is concerned, manufacturers in this space find themselves looking for a regulations-compliant, efficient data management solution capable of achieving improved traceability, enhanced target recalls, and better patient safety by leveraging the data every unique device identifier brings in.
In June 2020, the FDA issued an immediately-in-effect guidance on its policy regarding compliance dates for class I and unclassified devices that are not implantable, life-supporting or life-sustaining. This is an instance of a measure that indicates stronger controls over device surveillance and asks healthcare institutions to establish that the devices they use to dispense patient care are safe and feature in a centralized database.
An ongoing and effective data stream can help achieve these goals. It can also provide insights into the utilization of these critical assets, leading to their better management.
Want to learn more about why implementing the UDI is an integral part of the digital transformation of medical devices manufacturing organizations? Write to us at us-marketing@columbusglobal.com today!
